Indications
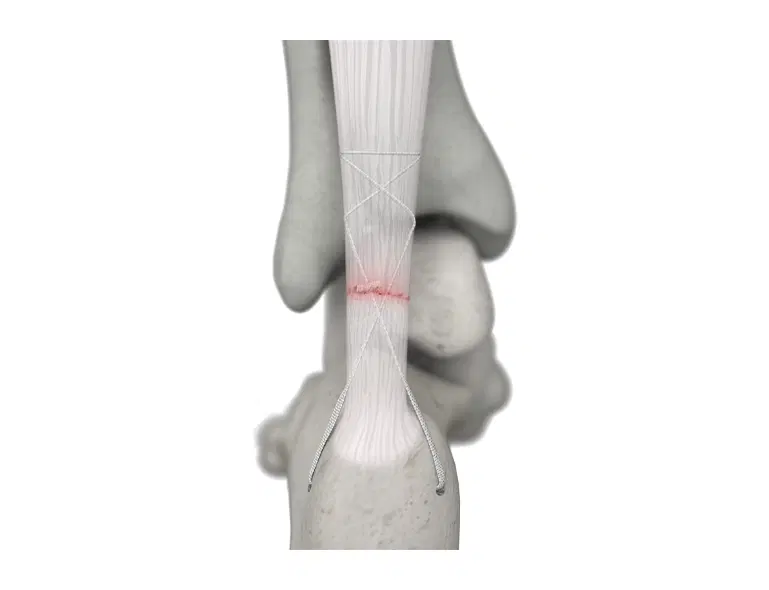
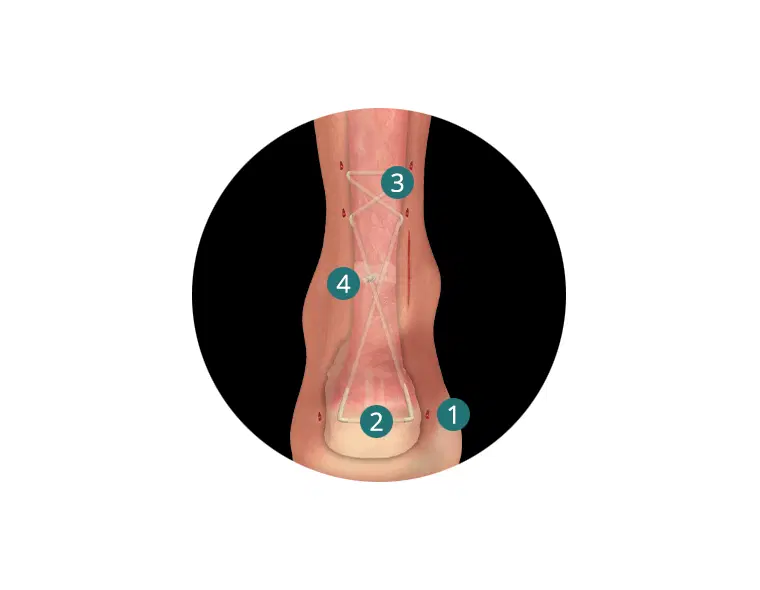
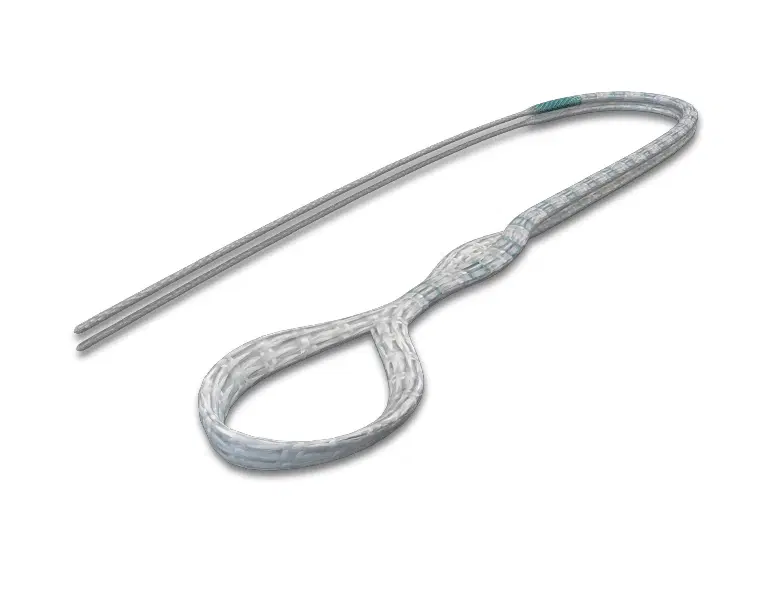
The AchilloCordPLUS™ is indicated for patients with acute ruptures of the Achilles tendon, but is particularly suited to patients where an extended period of postoperative immobilisation is undesirable.
Instructions For Use
To view the Instructions for Use please visit our IFU Library
AchilloCordPLUS System Implant Set Includes
AchilloCordPLUS System Implant Set, includes:
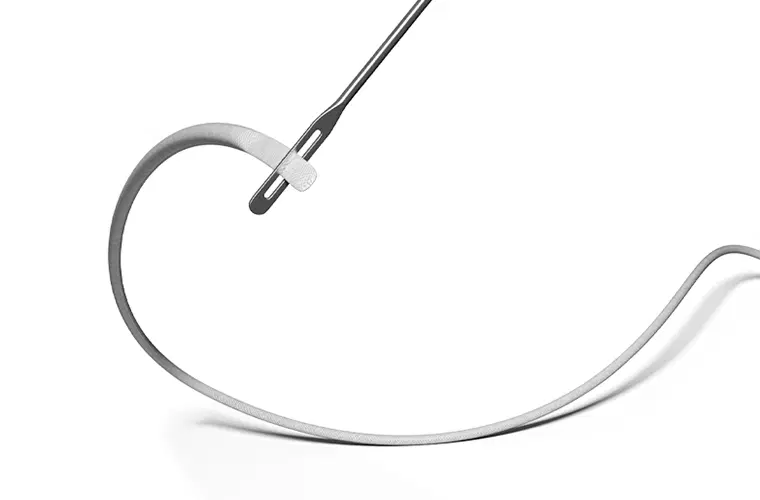
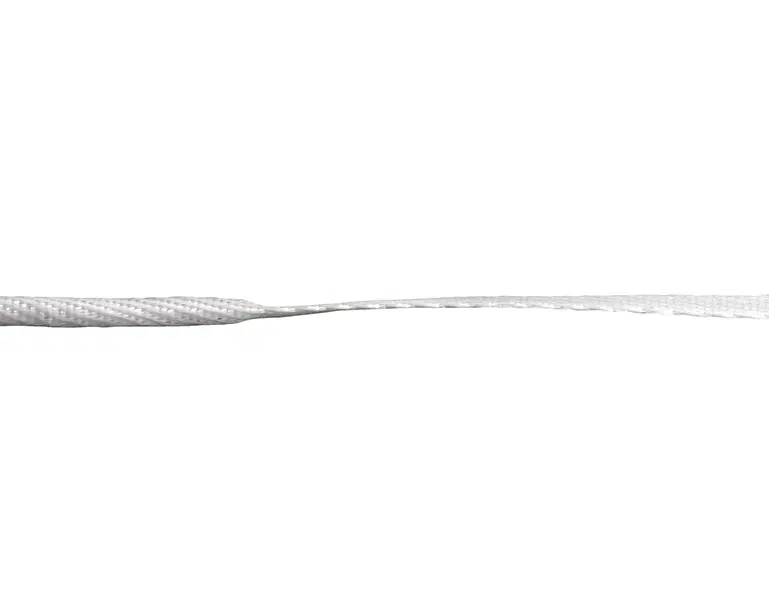
AchilloCordPLUS, 5 mm x 800 mm (supplied sterile)
Packaged with the following disposables:
Rigid Probe with eye, stainless steel, 20 cm (supplied sterile)
Drill Bit, plain shank to fit Jacobs Chuck, 3.2 mm diameter (supplied sterile)
Order Codes
102-1142 AchilloCordPLUS System Implant Set
202-3023 Rigid Probe